Harmful algal blooms (HABs) are a known and well-managed risk to seafood safety and human health, with extensive monitoring and mitigation measures in place, overseen by the NZ Seafood Safety Platform. However, HAB species can produce toxic compounds which can negatively affect the health of fish and shellfish that ingest or come into contact with the algae – with implications for the shellfish aquaculture industry.
The direct effects of HABs on cultured fish and shellfish species can be substantial and are likely to become more significant considering increasing climate anomalies and as the farmed supply of seafood grows.
Below we detail some of our latest HAB research produced through ShARP and explain how it links to our overarching objective to support the growth and resilience of New Zealand’s shellfish aquaculture industry.
1. HABs in a changing environment
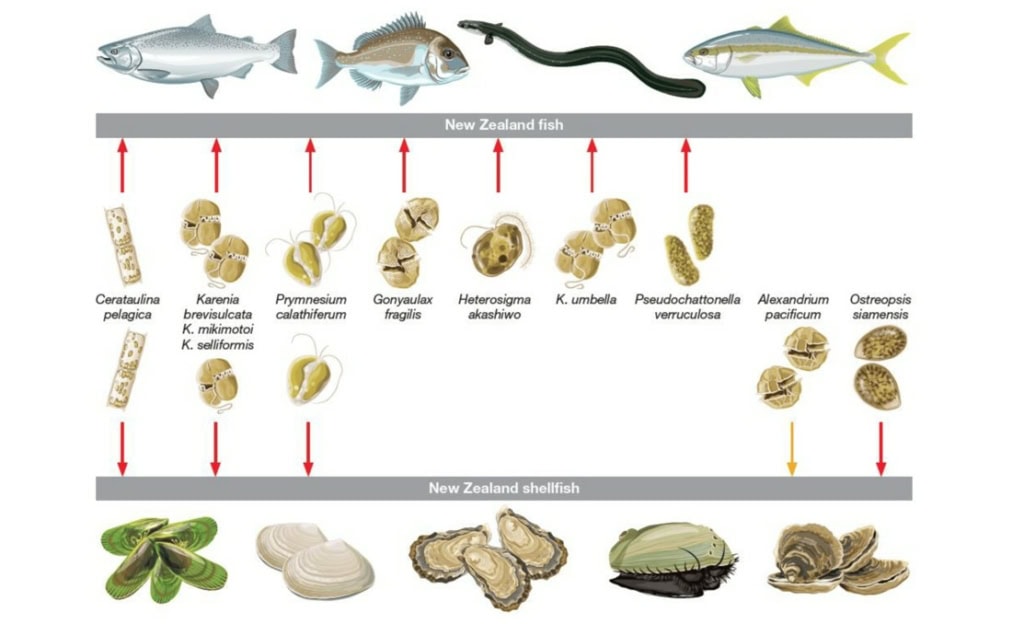
Recent salmon mortality, severe declines in greenshell mussel spat settlement, and unexplained summer mortalities of greenshell mussel have been reported in NZ. Although the causes of these events are not clear, HABs may be a contributing factor. Previous HAB events in NZ have impacted cultured species and HABs frequently occur in important growing areas for Pacific oysters, Green-lipped mussels, and Chinook salmon (Figure 1). Such events appear to be increasing in geographic range, duration, and frequency.

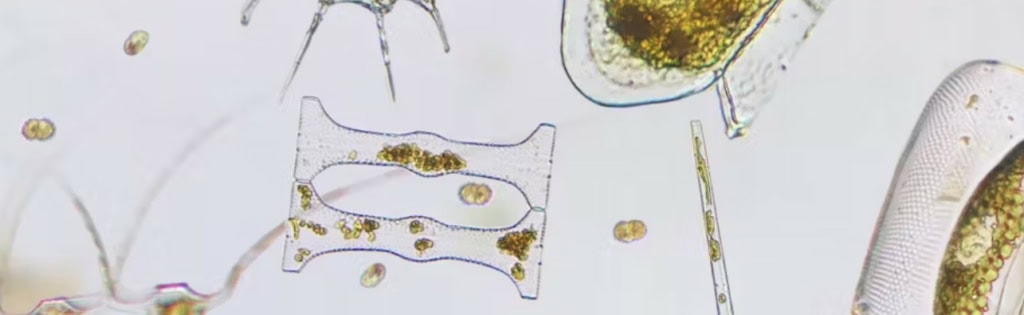
Figure 1a: Blooms of Pseudochatonella spp. in the Marlborough Sounds

Figure 1b: Blooms of Alexandrium spp. in the Marlborough Sounds
In a recent study lead by Dr Anne Rolton Vignier (Rolton et al. 2022), Cawthron researchers examined the HAB species which are known to bloom both globally and in NZ, their effects on commercially important fish and shellfish species, and what is likely to happen in the future as a result of our warming waters. (See recent news features on this article in Stuff and RNZ News.)
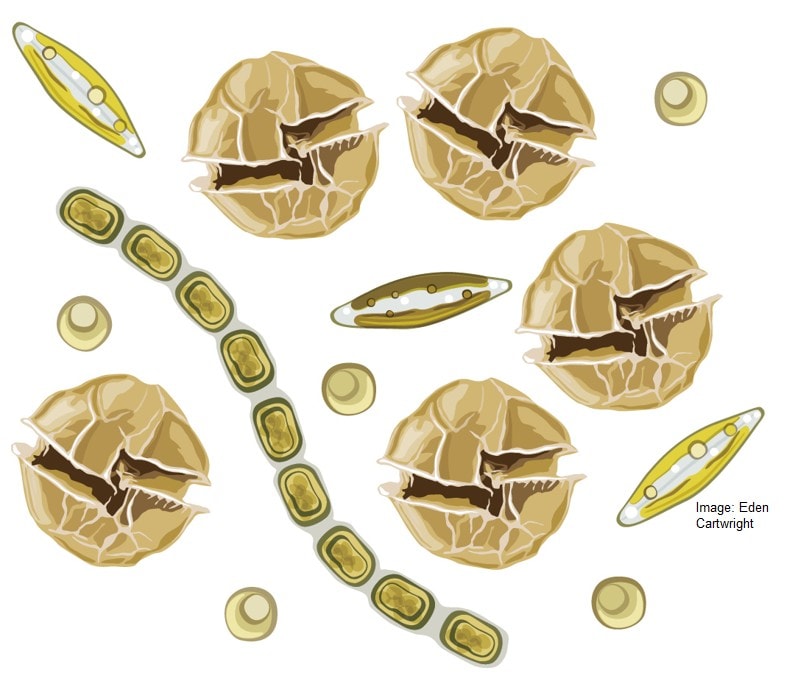
Gathering the most up-to-date research, reports, observations and drawing on our substantial HAB knowledge at Cawthron, we found that the HABs we have here can have a lot of very varied effects on fish and shellfish (Figure 2). These range from reducing feeding rates to inducing mortalities. One particular finding – backed-up by our latest research – was that cultured NZ species (i.e. Green-lipped mussels and flat oysters) which were previously thought not to be affected by blooms of A. pacificum actually can be significantly affected by these blooms (Figure 2).
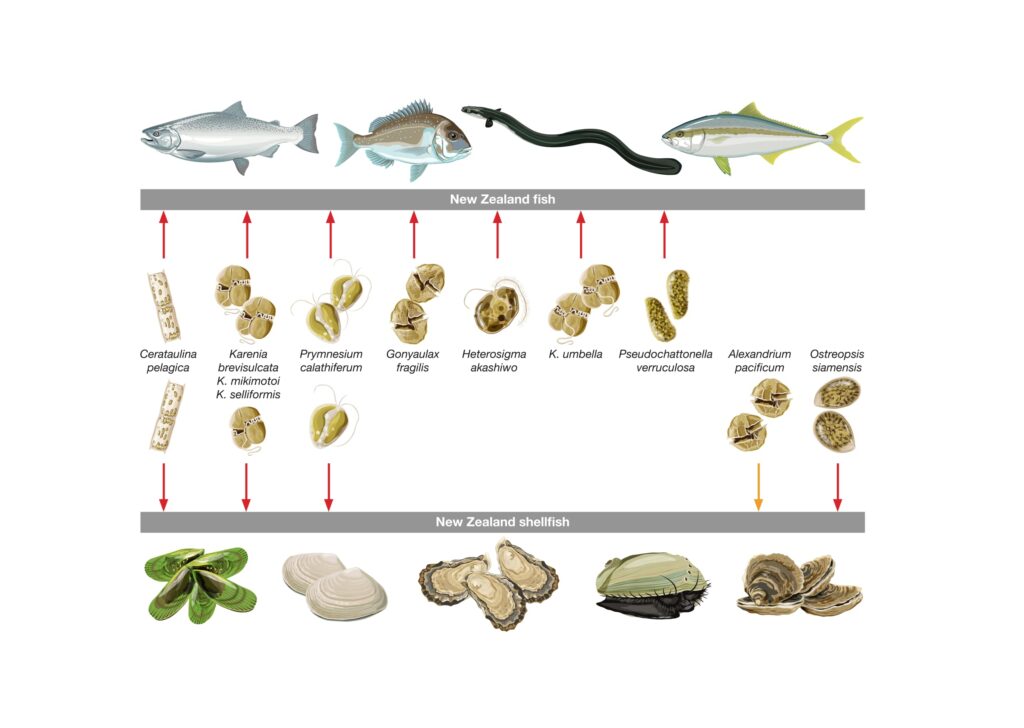
Figure 2. Known lethal (red arrows) and sublethal (orange arrow) effects of harmful algal species that have bloomed on fish and shellfish in New Zealand. Arrows indicate blooms that have impacted fish (e.g., Heterosigma akashiwo), shellfish (e.g., Alexandrium pacificum) or affected both fish and shellfish (i.e. Karenia brevisulcata). Image: Eden Cartwright
We also identified HAB genera and species that are already present in NZ and are likely to bloom in the future as a result of change in our waters, such as Karlodinium veneficum, Chatonella, Pfiesteria, Cochlodinium spp., as well as the emergence of novel HAB species to NZ such as Gambierdiscus and Alexandrium catenella, which are a major problem abroad.
As a response to our warming waters, we’re likely to see range expansions of HAB species, increases in bloom duration and intensity, and changes in HAB toxicity, combined with reduced resilience of fish and shellfish (Figure 3).
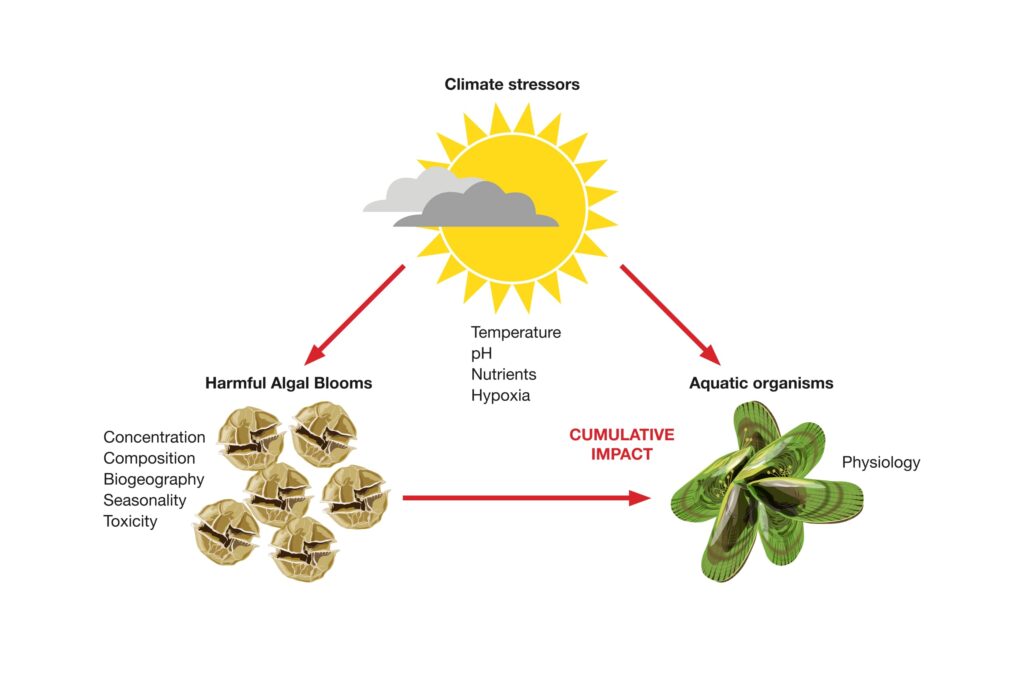
Figure 3. The effects of climate change stressors (e.g., temperature, pH, nutrients, hypoxia, salinity, turbidity, and anthropogenic stressors) on harmful algal blooms (HABs) and fish and shellfish species. Image: Eden Cartwright
This work serves as a useful HAB guide for both researchers and the aquaculture industry, providing a ‘heads-up’ for future HAB species and effects to look out for, and highlighting the importance of early detection, rapid HAB screening, testing local HAB strains and aquaculture species, and the need for research into the effects of HABs and other climate change related stressors to enable effective stock management.
2. Alexandrium blooms and greenshell mussels in the Marlborough Sounds
In the Marlborough Sounds, HABs of Alexandrium pacificum first appeared in Queen Charlotte Sound in 2011, in important Greenshell™ mussel growing and spat catching areas. Blooms have since spread westwards and now regularly bloom across the top of the South Island. In light of these recurrent Alexandrium blooms in the Marlborough Sounds, the PhD project of Hannah Greenhough (Cawthon Institute and University of Otago; Figure 4) is looking at “The effects of Alexandrium spp. exposure on green-lipped mussels and their reproductive processes”.

Figure 4: Hannah Greenhough cultures the HAB species Alexandrium pacificum to use in her experiments.
The early life stages of shellfish are known to be particularly sensitive to environmental stressors and our exposures of larval life stages of Green-lipped mussels to environmentally relevant concentrations of A. pacificum have resulted in substantial mortalities and reductions in larval growth (Figure 5). These effects appear to be unrelated to the paralytic shellfish toxins that cells of A. pacificum produce and more related to presence of other bioactive compounds associated with the algal cell (Greenhough et al. 2023).
Green-lipped mussels in NZ are exposed not only to high concentration blooms of A. pacificum lasting up to several weeks, but also to numerous stressors such as warming ocean temperatures, reduced salinity, acidification, sedimentation, pollutants and disease causing greater effects on shellfish.
Given the pressing need for this multiple stressor research, we also did exposures of green-lipped mussel spat to both low and high concentrations of A. pacificum AND low and high temperature (17 and 22⁰C). Exposure lasting only a few days, revealed strong additive effects of high temperature with high A. pacificum concentration on spat mortality and spat in this treatment continued to die after exposure had finished. Even surviving spat were severely affected during HAB exposure – being unable to put down byssus plaques which is their way to hold on to a surface like seaweed or ropes (Figure 6).
This work is on-going and will help farmers in the Marlborough Sounds with effective stock managements strategies.
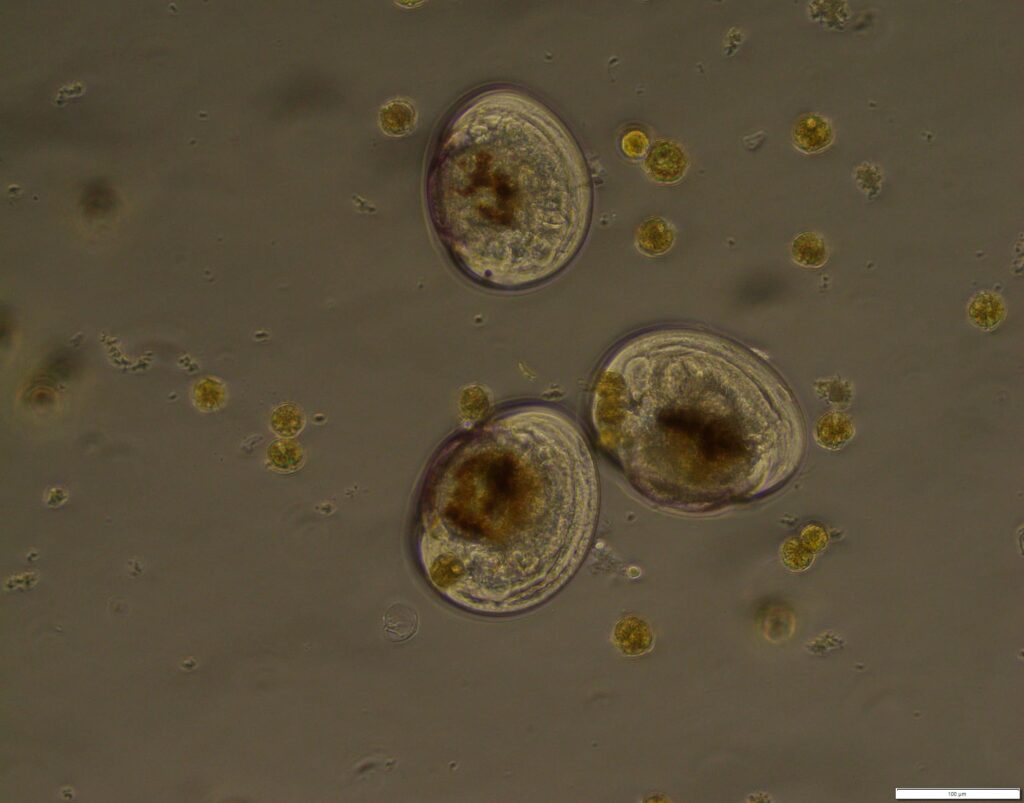
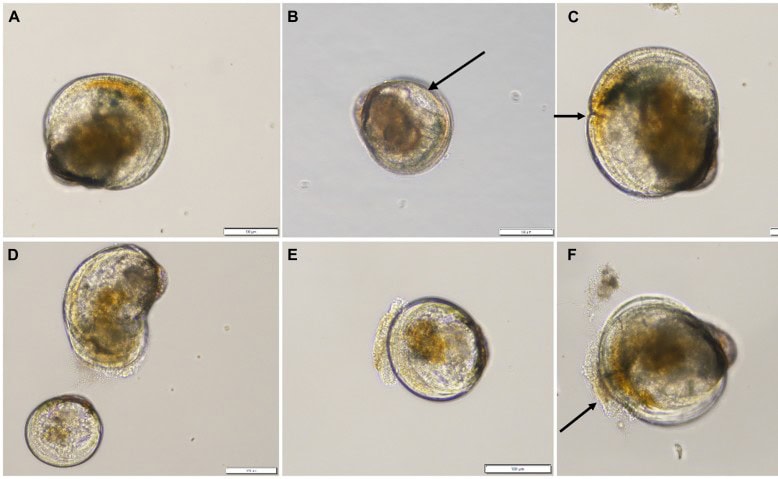
Figure 5: Cells of Alexandrium pacificum and green-lipped mussel larvae.
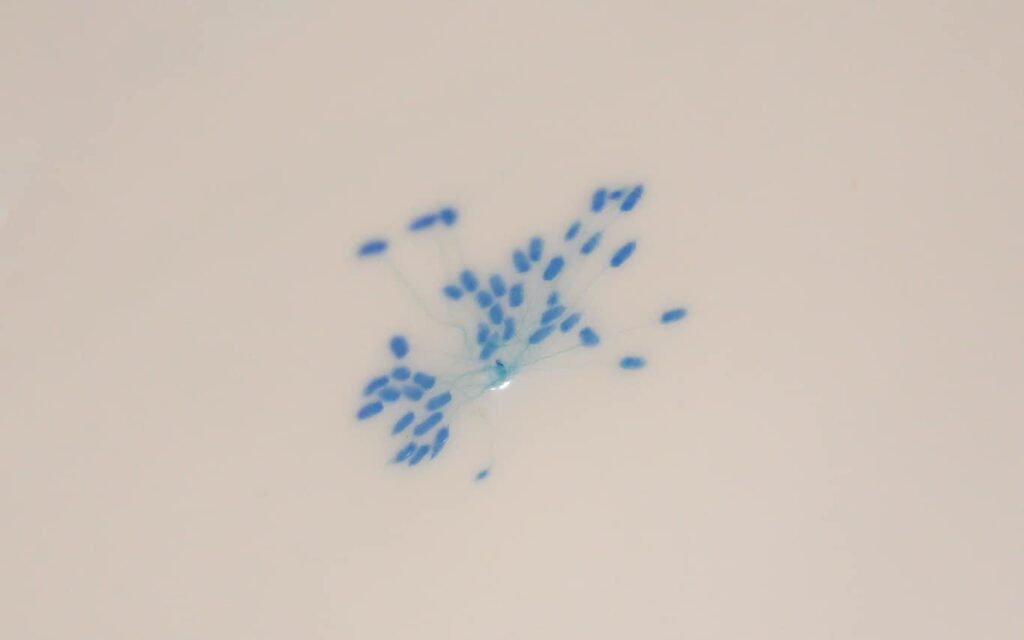
Figure 6: Byssal plaques stained with a dye ‘fast green’ to make them easier to count. These plaques are laid down by green-lipped mussel spat over 24h and are then counted after staining.
3. Developing bioassays for HAB toxin testing
Determining the effects of HABs on species of commercial interest can be time consuming and expensive. The aims of this project are to develop cell bioassays using fish gill cells and oyster gametes, instead of using whole animals, to test the toxicity of algal strains from NZ more easily (Figure 7). This will allow us to determine if HAB species are toxic to fish and shellfish species, quickly and relatively cheaply. For more information on this project see: Developing quick screening tests for harmful algal blooms (royalsociety.org.nz).
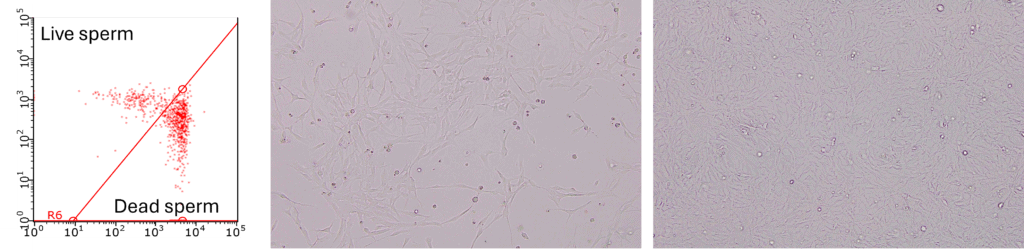
Figure 7: From left to right: oyster sperm exposed to Alexandrium pacificum, fish gill cells growing on the bottom of a cell culture flask, fish gill cells ready to use for toxic algal exposure.
Once established, these methods will be adapted to other relevant cell lines and applied to not just rapid tests for HAB toxins but, also many other toxic compounds and environmental stressors – helping provide rapid, accurate and cost-effective assessments of use to the aquaculture industry.

Contact Dr Anne Rolton Vignier
Senior Scientist and Ecotoxicology Leader – Cawthron Institute