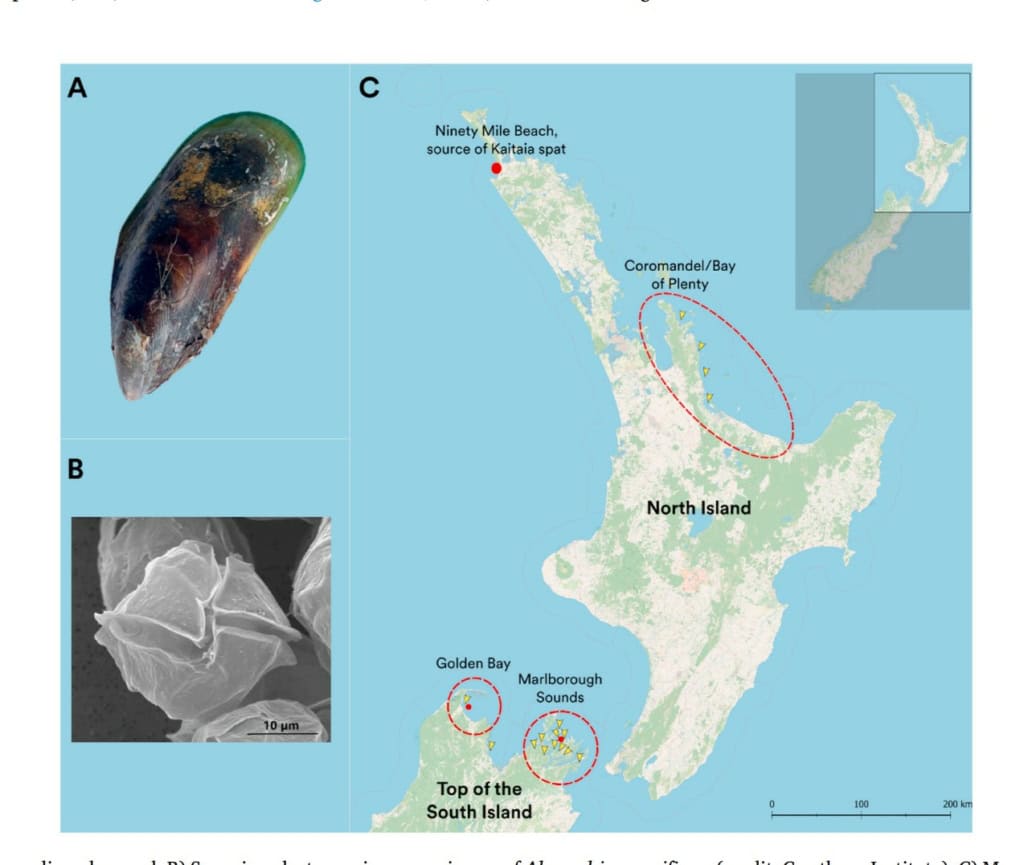
Summer die-offs of spat of Greenshell™ mussel/kūtai/green-lipped mussel (Perna canaliculus) and a severe decline in wild spat settlement have been reported in the Marlborough Sounds over the last decades, impacting seed supply and the industry’s ability to grow.
The causes of these effects are unknown, but we have preliminary evidence that deteriorating water quality – particularly the presence of heavy metals, biotoxins, and organic contaminants (e.g., pesticides) originating from land use activities – are contributing factors to the lack of settling larvae and unusual mortalities of spat. At the same time, rising temperatures along with an intensification of climate drivers (El Niño Southern Oscillations, Southern Annular Mode) and storm events are causing rapid and extreme changes to coastal environments. These climate-associated stressors (marine heatwaves, pH changes, and high sedimentation load and freshwater inputs) may exacerbate effects from contaminant inputs. A better understanding of these complex interactions in the Marlborough Sounds is essential to understand how we can prevent or mitigate these effects, and to help create knowledge that may help us prevent recruitment declines occurring in other regions like Northland which provides the vast majority of NZ’s kūtai spat (65% of the industry’s spat still originate from Te-Oneroa-a-Tōhē ǀ 90 Mile Beach).

Figure 1: Greenshell™ mussel/kūtai/green-lipped mussel (Perna canaliculus) newly settled spat on ropes.
Within the Shellfish Aquaculture Research Platform (ShARP) at Cawthron, we are using a three-pronged approach to tackle this issue.
1. Water quality monitoring in the Pelorus Sound
Supported by the industry (Marine Farming Association [MFA], Sanford, Clearwater Mussel Ltd) and academic collaborators (University of Waikato, University of Auckland, Plant & Food Research), a water quality monitoring scheme has been established since November 2021. Based on 40 years of mussel settlement data provided by the MFA, we selected several sites in the Pelorus Sound with contrasting levels of wild spat settlement. We used a passive sampling approach to characterize pollution levels in the actual surface waters at these three sites. Specifically, three different passive sampling devices (PSDs), including Diffusive Gradient Thin-Films (DGT), Chemcatchers™ and Solid Phase Adsorption Toxin Tracking (SPATT) samplers, which measure bioavailable metals, organic contaminants (e.g., pesticides) and biotoxins respectively, were deployed for several weeks across our three study sites: Garnes Bay, Beatrix Bay, and Skiddaw (Fig. 2).
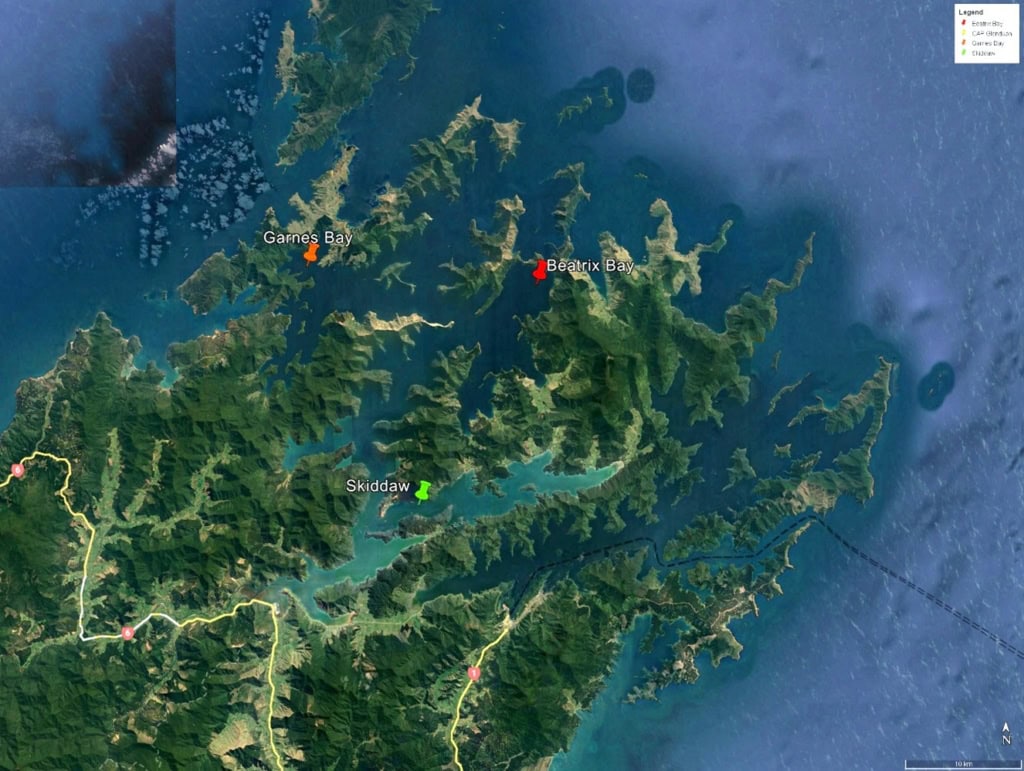
Figure 2: Study area – the Pelorus Sound.
In addition, tissue from resident mussels were periodically collected from each site to quantify the uptake and bioaccumulation of contaminants. Sediment samples were also collected at each site as a complementary ‘multi-compartment’ approach.
Early findings show elevated levels of certain heavy metals (notably Zinc) during the mussels’ recruitment peak, which appear to coincide with heavy rain events. Furthermore, a cocktail of biotoxins and organic contaminants, including herbicides, were extracted from the rest of the PSDs deployed in the field. Variation over time and season were observed, with some key toxins predominantly present during late summer and overlapping with the spawning and recruitment season.
This pioneering approach in water quality monitoring, combined with the new testing approaches below, can provide valuable environmental data to help us better understand the drivers behind declines in wild spat recruitment and mortalities in the region.
2. Development of bioassays using early life stages
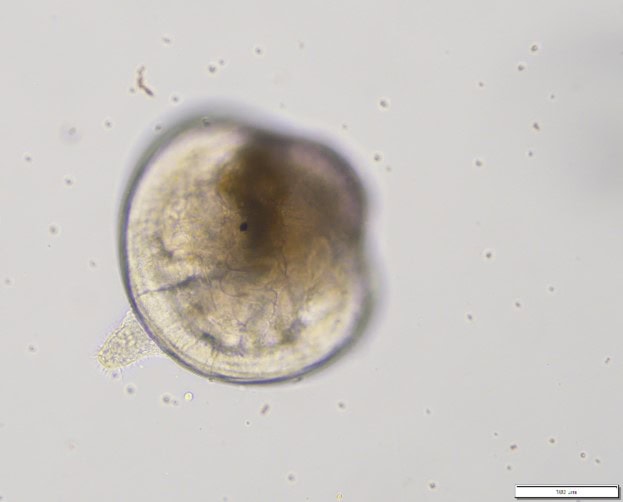
In marine bivalves, planktonic early life stages such as sperm, eggs, embryos and larvae, which are released into the water to undergo their development, are more sensitive to stressors and pollutants than adult stages. As a result, embryos are valuable tools in ecotoxicological assessments. Despite the commercial and ecological significance of Greenshell™ mussels, currently, in NZ, most studies that look at the effects of contaminants are carried out using embryos of the blue mussel (Mytilus galloprovincialis), which may not respond in the same way as Greenshell™ mussel early life stages. Our previous hatchery research suggests that the developing Greenshell™ mussel embryos and larvae may be particularly sensitive to changes in water chemistry (McDougall et al. 2020; French et al. 2021). We focused on developing reliable and sensitive lab-based bioassays to study the toxicity of contaminants on the early life stages (sperm, embryos and larvae) of Greenshell™ mussels and compared their sensitivity with the NZ and international standard – the blue mussel (Mytilus galloprovincialis).
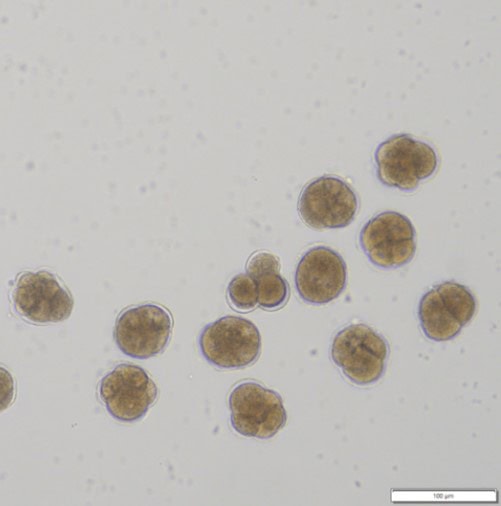
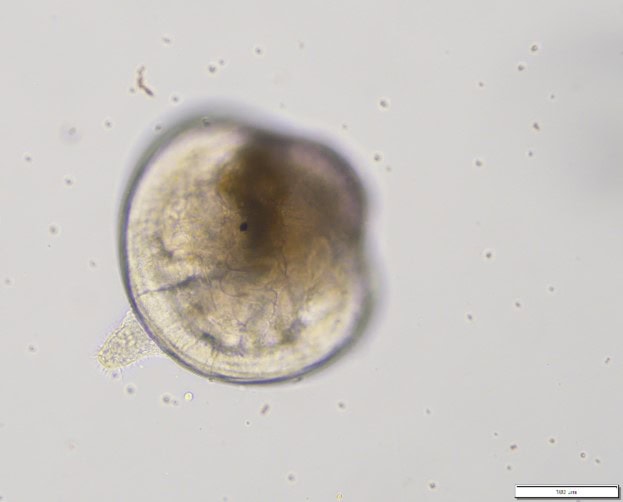
Figure 3: (left) Kūtai/Greenshell™ mussel (Perna canaliculus) fertilised eggs and (right) competent 20-day old larva before settlement.
- Spermio-toxicity assay
Two reference contaminants; the metal Zinc, and the antimicrobial agent Triclosan, were used to expose sperm of Blue mussels and Greenshell™ mussels and develop a rapid ecotoxicity test: the spermio-toxicity assay. Following 1 hour of sperm exposure, a range of parameters were investigated including sperm cellular characteristics via flow cytometry, fertilization success, and subsequent embryo and larval mortality. Overall, our results showed (i) that the endemic Greenshell™ mussel has similar sensitivity to contaminants as blue mussels, and that (ii) Greenshell™ mussel sperm can be successfully used to rapidly assess the toxicity of chemicals (Rolton et al. 2022).
- Embryo and larval development assay
Last year (2023) marked a breakthrough in our ecotoxicological research. We have been able to conduct an embryotoxicity assay with Greenshell™ mussels in artificial seawater without using EDTA, a metal chelator (which binds metals) previously thought to be indispensable for successful embryo and larval development of Perna. This means we can now use Greenshell™ mussel embryos (Figure. 3 left) to test ecotoxic effects of any contaminants, including heavy metals, and compare the sensitivity of Greenshell™ mussels to pollutants with the international standard, the blue mussel (Mytilus galloprovincialis).
- Larval settlement assay
The team has further optimised and validated a settlement assay using competent larvae of Greenshell™ mussels (Figure. 3 right). Based on field-levels of trace metals measured from the DGT samplers in the Sounds, Greenshell™ mussel larvae were exposed for 96h to increasing concentrations of Zinc and Lead. We found that levels of bioavailable Zinc high enough to be detrimental to settlement, could be occasionally reached in the field and our hatchery water supply, whereas toxic thresholds for Lead were above those detected in the field. For the full details of the study see Vignier et al. 2024.
3. Assessing the toxicity of environmentally relevant contaminants in a climate change scenario
The toxicity of the different cocktails of biotoxins and organic contaminants (including herbicides), extracted from field-deployed PSDs, was evaluated by using our spermiotoxicity assay developed last year. Sperm were exposed for 1 hour to various extracts obtained from the PSDs, and viability of the sperm cells was assessed on the flow-cytometer, and their subsequent ability to fertilise eggs was examined. We found some extracts were non-toxic and some were highly toxic to sperm cells, depending on the extracts tested. The use of in-vitro toxicity assessment using sperm cells and flow-cytometry show great promise as a screening tool to establish reproductive toxicity of environmentally relevant contaminants.

Figure 4: (left) Biotoxin extract collected from the field to be tested on Greenshell™ mussel sperm (spermiotoxicity assay); (right) Guava flowcytomer used for high-throughput cellular assessments.
Finally, additional funding has enabled us to strongly leverage this research and further investigate how climate change can exacerbate the impacts of marine pollution on Greenshell™ mussels. This new grant will allow us to (i) continue our environmental monitoring scheme in the Sounds, and (ii) expand our lab-based investigations into the combined effects of temperature, contaminants and field-collected sediments on Greenshell™ mussel fitness. For more detailed information on this research, see this publicly available report to Ministry of Primary Industries.

Contact Dr Julien Vignier
Senior Scientist – Hatchery techniques and ecotoxicology – Cawthron Institute